Two research teams from the Energy Geosciences Division (EGD) at Berkeley Lab have been selected to receive a total of $3.24 million in funding from the U.S. Department of Energy Advanced Research Projects Agency-Energy (ARPA-E) to study the potential of geologic hydrogen as a clean energy source. The funding is part of a first-of-its-kind R&D program focused on stimulating the generation of hydrogen through chemical reactions between iron-rich silicate rocks in the Earth’s crust and water underground. The hydrogen formed in this way can provide carbon-free energy at the scale needed for a substantial clean-energy transition.
These two projects are among 16 announced Thursday by DOE as recipients of a total of $20 million for research supporting efforts to enable commercial-scale deployment of clean hydrogen at the lowest cost and environmental impact. EGD scientists work to understand the behavior of fluids in underground rock and have applied this knowledge to developing geologic carbon storage and geothermal energy technologies — and now to generating and extracting hydrogen from Earth’s subsurface through chemical, hydrologic, and mechanical processes, safely, and at scale.
“We are excited about the enormous opportunity that exists to explore the emerging field of geologic hydrogen. These two awards will help tackle some of the significant challenges that remain before this promising source of low-carbon energy can achieve widespread use,” said Jens Birkholzer, the Director of Berkeley Lab’s Energy Geosciences Division.
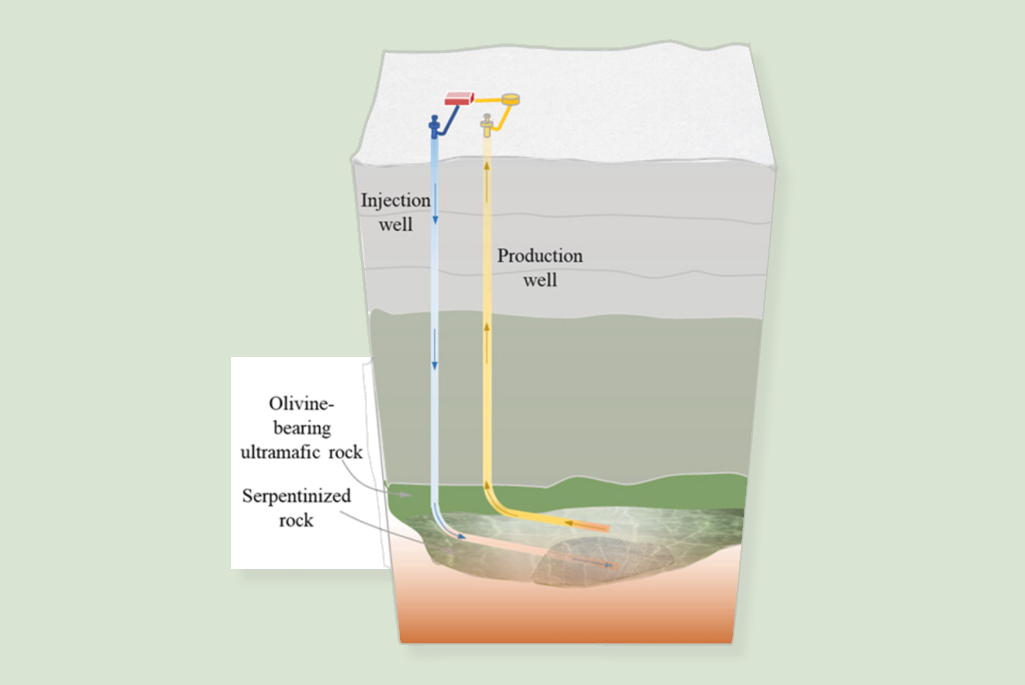
Locked within certain iron-rich silicate rocks in Earth’s subsurface, especially the mineral olivine, is a chemical reactivity sufficient to produce hydrogen upon contact with water. Iron is released from the rock via a process known as serpentinization. This resource can be unlocked by injecting water into fractures in underground rock, initiating chemical reactions to evolve hydrogen, and then developing methods to efficiently extract the hydrogen that is generated by the serpentinization process.
Berkeley Lab Research Scientist Mengsu Hu will lead the project Cyclic Injection for Commercial Seismic-Safe Geologic H2 Production (CyclicGeoH2) in collaboration with UC Berkeley and the University of Texas at Austin. Awarded $2.0 million, it is one of six projects to receive awards for research focusing on technologies related to the extraction of geologic hydrogen. These teams will work to improve underground transport methods and engineered containment, reservoir monitoring, and/or modeling during hydrogen production and extraction, as well as assessing the risk of hydrogen reservoir development.
Hu’s project team will develop a technology that aims to address two key challenges of geologic hydrogen production: extracting hydrogen both safely and economically at commercial scale. These include evaluating where and how deep to generate and extract hydrogen, how to maximize the generation rate, and how to avoid the loss of hydrogen due to leakage or inefficient extraction.
The technology is based on cyclic injection of fluids at different pressures, temperatures, and pH to control the extraction of H2 without inducing harmful seismicity. Their approach involves adaptive controls of fracture creation, serpentinization and associated volumetric expansion and induced seismicity, and extraction of hydrogen to the wellhead. Using rock samples from field sites in Montana and elsewhere, the team will apply an integrated approach including multiscale numerical modeling, laboratory tests, and field characterization to develop and test the novel technology.
The project team consists of Lead PI Mengsu Hu (LBNL), Co-PIs Carl Steefel (LBNL), Michael Manga (UC Berkeley), Jonny Rutqvist (LBNL), Nicolas Espinoza (UT Austin), John Foster (UT Austin), Wenming Dong (LBNL), and Seiji Nakagawa (LBNL), and project administrator Reed Helgens (LBNL).
Berkeley Lab Senior Scientist Ben Gilbert will lead the project, Orange Hydrogen from Catalytic Low-Temperature Serpentinization. Awarded $1.24 million, it is one of 10 projects to focus on improving geologic hydrogen production by speeding up the rate of chemical reactions at low temperature. Although hydrogen production increases under high temperatures (200-350 C), these temperatures exist at depths that are costly and commercially risky to access. Catalytic hydrogen generation from low-temperature serpentinization, however, would enable huge volumes of shallow and cool ultramafic rock to be exploited for hydrogen production.
Gilbert’s team will use quantum chemistry simulations and experiments to uncover mechanisms for accelerated and sustainable low-temperature serpentinization. The project is a collaboration between LBNL scientists, Ben Gilbert, Piotr Zarzycki, Harry Lisabeth and Eric Sonnenthal, UC Berkeley graduate student Yarong Qi, and Drew Syverson at the University of North Carolina at Charlotte. If the early-stage research is successful, insight on translating this research to candidate sites in the U.S. will be provided by U.S. Geological Survey scientists Chris Jenkins and Mike Zientek.
The researchers will explore the three spontaneous reactions that occur when iron-bearing silicate rocks are exposed to water: hydration of the iron-bearing silicate minerals, the hydrogen evolution reaction, and the capture of iron by minerals formed through the serpentinization reactions. Their goal is to show that these reactions can be understood, predicted, and controlled in the laboratory and ultimately the field. The team will seek to patent and commercialize the approaches and substances shown to control these reactions.